COLLIGATIVE PROPERTIES OF SOLUTIONS
Objective : to investigate the colligative properties of non-electrolytes and electrolytes solutions
When a non-volatile (substances that do not readily form vapors), non-electrolytic (substances that do not form ions and do not conduct electricity when placed in water) solute such as sugar is dissolved in a given volume of solvent to form a sugar solution, it changes the set of properties of the pure solvent entirely. In this regard, the set of properties such as freezing point, boiling point, vapor pressure, and osmotic pressure of a solvent are affected by the presence of the solute particles in the solution. This set of properties will depend only on the number of dissolved particles in the solution and not on their identity. These properties are collectively known as colligative properties of solution.
This module will focus on four colligative properties of electrolyte and non-electrolyte solutions namely:
· vapor pressure lowering,
· boiling point elevation,
· freezing point depression,
· osmotic pressure.
| The Colligative Properties of Solution | Mathematic equation for | |
| Non-electrolytes Solution | Electrolytes Solution | |
| Vapor pressure lowering (1st Raoult’s Law) | 
| 
|
| Boiling point elevation (2nd Raoult’s Law) | 
| 
|
| Freezing point depression (2nd Raoult’s Law) | 
| 
|
| Osmotic pressure (Van’t Hoff law) | 
| 
|
There are:
· Esvt is boiling point elevation constant, equivalent to 0.52 ° C/m for aqueous solutions. This means that, for example, 1 mole of sugar (non-electrolyte) in 1 kilogram of water will increase the boiling point from 100°C to 100,52°C. And Cm is the molal concentration of solute.
· Ksvt is the freezing point depression constant equivalent to -1,86°C/m for aqueous solutions. Again, for example, 1 mole of sugar (non-electrolyte) in 1 kilogram of water will decrease the freezing point from 0°C to -1,86°C.
·  The i Van’t Hoff factor gives the number of particles per formula unit of the electrolyte solute. The degree of dissociation is associated with an isotonic factor by next ratio:
The i Van’t Hoff factor gives the number of particles per formula unit of the electrolyte solute. The degree of dissociation is associated with an isotonic factor by next ratio:
1st Experiment: I nvestigate and c lassify colligative and non-colligative properties of solution
Materials and Reagents: 1% (w/v) brown sugar solution, 0.5 % (w/v) salt solution (NaCl), thermometer
Procedure:
Prepare the brown sugar and salt solutions of concentration 1% and 0.5% respectively. Observe the following properties in the two solutions and identify whether the property observed is a colligative or non-colligative property based on the definition.
|
|
|
| № | Properties of Solution | 0.5 % (w/v) salt solution | 1% (w/v) brown sugar solution |
| 1 | Solubility (ability of the solute to be dissolved in a solvent) | ||
| 2 | Viscosity of solution (“viscosity” is a property of solution to resist flow) | ||
| 3 | Color of the solution | ||
| 4 | Taste of solution | ||
| 5 | Temperature upon boiling | ||
| 6 | Temperature upon freezing |
Analysis:
1. Which of the following properties of solution can be considered colligative? Why?
2. Which of the following properties of solution can be considered non-colligative?
Justify your answer.
2nd Experiment: I nvestigate the vapor-pressure lowering of non-electrolyte sugar solution
Materials and Reagents: water, aqueous sugar solution, two glasses, one sealed enclosed container
 Procedure:
Procedure:
1. Get two glasses placed side by side in a sealed enclosed container. One glass contains pure water, the other an equal volume of an aqueous solution of sugar. Take note of the volume of water and sugar solution. (See figure 3.1a)
2. Leave the set-up until the next day. Gradually measure the volume of the sugar solution and that of the pure water. Is there any change in their original volume? (See figure 3.1b).
After finishing the activity, you should now see why the vapor pressure of the solvent decreases upon addition of a non-volatile, non-electrolyte solute (sugar).
Analysis:
1. What can you infer about the change in volume of the sugar solution and that of pure water?
2. What has caused this change in volume of the sugar solution and that of pure water?
3rd Experiment: I nvestigate the Boiling Point Elevation
Materials and Reagents: three eggs, 1 tea spoon of salt, 1 tea spoon of sugar, boiling water.
PROCEDURE:
1. Put the first egg in two cups of water and take note of the time until the water boils.
|
|
|
2. Again, using two cups of water, put the second egg in the water and add 1 tbsp of salt. Record the time it will take the water to boil.
3. Repeat step 2, but add 1 tbsp of sugar instead of salt. Again, take note of the time it will take the water to boil.
4. Compare the time it will take for water to boil and cook the hard-boiled egg in step 1 to step 3. Record your observations.
Analysis:
1. Which set-up took less time to cook hard-boiled eggs? Why?
2. Did the water take more time to boil when electrolyte (NaCl) solute was added? Why?
3. Did the water take more time to boil upon addition of non-electrolyte (sugar) solute? Justify your answer.
4th Experiment: I nvestigate the Freezing Point Depression
Materials and Reagents: one tsp. salt, one glass of water, one glass of crushed Ice, stirrers
Spoon, thermometer
PROCEDURE:
1. Using one glass of water and one glass of crushed ice, stir the mixture; then using a thermometer, observe and record this temperature.
2. Add one tsp. of salt to the water/ice mixture, then observe and record the temperature. You should repeat this procedure until the temperature reaches 10oC. More ice should be added if it is necessary.
Analysis:
1. What happens to water and ice when salt is added to this mixture?
2. What happens to the temperature when salt is added to the mixture?
3. What variables would cause these differences?
EXERCISES:
1) What is the osmotic pressure of the solution containing 0.2 M sugar (C12H22O11) solution at 25oC?
2) At what temperature will the sugar solution boil and freeze if 20 g sucrose (C12H22O11) is added to 1.5 kg of water?
3) What is the boiling and freezing points of a 0.20 m NaCl solution in water? For water, E is 0.512oC*kg/mol; K is 1.86oC*kg/mol
4) Choose the letter of the best answer. Write the chosen letter on a separate sheet of paper:
| № | Question | Answer |
| 1 | A substance whose water solution conducts an electric current is called a(n) | a. electrolyte b. nonelectrolyte c. polar substance d. nonpolar substance |
| 2 | A substance that vaporizes easily is known as | a. volatile b. nonvolatile c. electrolyte d. nonelectrolyte |
| 3 | Adding a solute such as NaCl to water increases its | a. boiling point b. melting point c. osmotic pressure d. vapor pressure |
| 4 | Which of the following is NOT a colligative property of solution? | a. solubility b. boiling point c. melting point d. osmotic pressure |
| 5 | Which of the following statements is TRUE about vapor pressure? | a. The vapor pressure of the solvent is less than the solution. b. The vapor pressure of the solution is higher than the pure solvent. c. The vapor pressure of the pure solvent is higher than the solution. d. The vapor pressure of the pure solvent and the solution are the same. |
| 6 | The i factor gives the number of particles per formula unit of the solute. What is the i factor for NaCl? | a. 0 b. 1 c. 2 d. 3 |
| 7 | What happens during osmosis? | a. Pure solvent diffuses through a membrane but solutes do not. b. Pure solutes diffuse through a membrane but solvent does not. c. Gases diffuse through a membrane into a solution and build up pressure. d. Pure solvent and a solution both diffuse at the same time through a membrane. |
| 8 | What is the temperature at which the vapor pressure equals the atmospheric pressure? | a. 100oC b. freezing point c. boiling point d. melting point |
| 9 | The freezing point of a solution is always __________ the pure solvent. | a. less than b. same as c. greater than d. equal to the freezing point of solvent |
| 10 | What is the van’t hoff factor for CaCl2? | a. 0 b. 1 c. 2 d. 3 |
| 11 | According to 1st Raoult's law, which statement is FALSE? | a. The vapor pressure of a solvent over a solution decreases as its mole fraction increases. b. The solubility of a gas increases as the temperature decreases. c. The vapor pressure of a solvent over a solution is less than that of pure solvent. d. The greater the pressure of a gas over a solution the greater its solubility. |
| 12 | Which of the following statement is TRUE about the boiling point of the solution? | a. The boiling point of the solvent is greater than that of the solution. b. The boiling point of the solution is less than that of the pure solvent. c. The boiling point of the solution is the same as that of the pure solvent. d. The boiling point of the solution is greater than that of the pure solvent. |
| 13 | The boiling point of an impure compound is generally __________ that of pure solid. | a. same as b. less than c. greater than d. equal to the boiling point of pure solid |
| 14 | The melting point of an impure compound is generally __________ that of the pure solid. | a. less than b. the same as c. greater than d. equal to the boiling point of pure solid |
| 15 | Adding sodium chloride to water will cause the: | a. boiling point to rise and the freezing point to lower. b. boiling point to lower and the freezing point to rise. c. both boiling point and freezing point to rise. d. both boiling point and freezing point to lower. |
| 16 | Consider a solution made from a nonvolatile solute and a volatile solvent. Which statement is TRUE? | a. The osmotic pressure is the same as vapor pressure of the solution. b. The vapor pressure of the solution is always greater than the vapor pressure of the pure solvent. c. The boiling point of the solution is always greater than the boiling point of the pure solvent. d. The freezing point of the solution is always greater than the freezing point of the pure solvent. |
| 17 | Dissolving a solute such as NaCl in a solvent such as water results in: | a. an increase in the melting point of the liquid b. a decrease in the boiling point of the liquid c. a decrease in the vapor pressure of the liquid d. no change in the boiling point of the liquid |
5) TRUE or FALSE. Write TRUE if the statement is correct. Otherwise, write FALSE.
|
|
|
|
|
|
__________ 1. Colligative properties will only depend on the number of dissolved particles in the solution and not on their identity.
__________ 2. The addition of a solute will increase the boiling point of the solution.
__________ 3. The van’t Hoff factor is only applicable for non-electrolyte solutions.
__________ 4. The boiling point of the solution is always greater than that of the pure solvent.
__________ 5. All biological membranes are regulated via osmosis.
6) Match the correct equation in Column B with the principles in Column A.
| Column A | Column B | Your answer |
| 1. Raoult’s law | a. ΔTb = Eb*Cm | |
| 2. Dalton’s law of partial pressures | b. P = CMRT | |
| 3. Boiling point elevation | c. P1= Po1 * X1 | |
| 4. Freezing point depression | d. Ptotal = PA + PB | |
| 5. Osmotic pressure | e. ΔTf = Kf *Cm | |
| f. ΔP = P2 - P1 |
LABORATORY WORK
IONIC REACTION
Objective : investigate the reactions of ions in solution
· Students understand terms such as double displacement reactions & different types of such reactions like neutralization, precipitation.
· Students classify the compounds that give double displacement reactions.
· Students acquire the skill to perform a double displacement reaction using barium chloride and sodium sulphate.
· Students will be able to distinguish a double displacement reaction from a given set of chemical reactions in future.
The purpose of these experiments is to:
· write balanced equations
· study ionic reactions
· write net ionic reactions
Materials and Reagents : test tubes, indicators, electrolytes solutions
Ions are atoms (or groups of atoms) that carry electrical charge. Positively charged ions are called cations, whereas negatively charged ions are called anions.
Ionic compounds have certain properties common to all of them:
1) High melting and boiling points (they are non-volatile)
2) Soluble in water. Water is polar solvent and tends to dissolve ionic compounds. Soluble ionic compounds dissociate into ions in water (This process is called ionic dissociation):

3) Solutions of ionic compounds conduct electricity. Conduct electricity when molten or in aqueous solution: solid ionic compounds do not conduct electricity, when the substance is melted or dissolved in water, however, the ions move freely and carry an electric current.
4) Ionic crystals shatter easily.
| COMPOUNDS | ||
| Electrolytes are substances that dissociate into ions when dissolved in water and conduct electricity | Nonelectrolytes are substances that do not form ions and do not conduct electricity when placed in water | |
| Strong electrolytes : | Weak electrolytes : | |
| · completely dissociate into ions, · solutions strongly conduct electricity, · typical compounds: soluble ionic compounds (salts), strong acids and alkalies | · incompletely dissociate into ions, · solutions weakly conduct electricity, · typical compounds: weak acids and bases, slightly soluble and insoluble salts | · no dissociation, · solutions don't conduct electricity, · typical compounds: molecular organic compounds |
Acids are substances that increase the concentration of H+ when dissolved in water (by Arrhenius theory) or proton donors (by Bronsted–Lowry theory). There are only seven strong acids:
· Hydrochloric (HCl)
· Hydrobromic (HBr)
· Hydroiodic (HI)
· Nitric (HNO3)
· Sulfuric (H2SO4)
· Chloric (HClO3)
· Perchloric (HClO4)
Bases are a substances that increase the concentration of OH− when dissolved in water or proton acceptors. Strong bases are group 1 hydroxides (soluble bases are called alkali: NaOH, KOH) and soluble group 2 hydroxides (Ca(OH)2, Ba(OH)2, Sr(OH)2)
Neutralization Reactions occurs when a solution of an acid and a base are mixed and as a result forms a salt and water:
HCl(aq) + NaOH(aq) ® H2O(l) + NaCl(aq)
The term neutralization is often used to describe a reaction in which equal amounts of acid and base react with each other. Acid and bases can react with some compounds to change their color, these compounds are dyes that change color as the pH changes are referred to as indicators. This is one method used to determine the point at which exact amounts of acid and base have been reacted in neutralization reactions.
Strength of the electrolyte is described by the following values: the degree (a) and the dissociation constant (Kdiss) of the electrolyte. Degree of ionization 'a' may be defined as a fraction of total number of molecules of an electrolyte which dissociate into ions. The arbitrary border between weak and strong electrolytes is 30 % dissociation in solution.


where n – Number of molecules dissociated as ions, N – Total number of molecules of electrolyte dissolved. For strong electrolytes a>0,3 (30%), and for weak electrolytes a<0,3 (0 – 30%).
Ions present in solution constantly re-unite to form neutral molecules and, thus, there is a state of dynamic equilibrium between the ionized the ionized and non-ionised molecules, i.e.,
AxBy 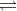 x A+ + y B-
x A+ + y B-
Applying the law of mass action to above equilibrium:

where K is known as dissociation (ionization) constant. The electrolytes having high value of Kdiss are termed strong electrolytes and those having low constant value of Kdiss as weak electrolytes.
A chemical equation which shows dissociation of electrolyte and written as dissociated ions of electrolyte is known as ionic equation.
Double displacement reactions may be defined as the chemical reactions in which one component each of both the reacting molecules is exchanged to form the products . During this reaction, the cations and anions of two different compounds switch places, forming two entirely different compounds. The general equation which represents a double displacement reaction can be written as:

• Metathesis reactions will lead to a change in solution if one of three things occurs:
– an insoluble solid is formed (When a chemical reaction forms such a solute, the insoluble solute comes out of solution and is called a precipitate),
– weak or nonelectrolytes are formed, or
– an insoluble gas is formed (H2S, CO2, SO2, NH3).
Let’s write one balanced ionic equation for one of the precipitate reaction between next electrolytes in their aqueous solution.
1) First write the balance chemical molecular equation:
(NH4)2S + Cd(NO3)2 ® 2NH4NO3 + CdS
2) Then write the total ionic equation by determining the dissociation of reactants and products in solution. Remember, a strong electrolyte is written as ions while a weak electrolyte is written as molecule because it cannot be dissociate completely in solution. Total ionic equation includes only those compounds and ions that undergo a chemical change in a reaction in an aqueous solution. (Not spectator ions):
Cd2+ + 2NO3- + 2NH4+ + S2- ® CdS¯ + 2NO3- +2NH4+
Spectator ions are ions that didn’t take part in a chemical reactions. They are found on both sides of the reaction. Spectator Ions NO3- and NH4+ , therefore must be excluded in net ionic equation
Cd2+ + 2NO3- + 2NH4+ + S2- ® CdS¯ + 2NO3- +2NH4+
3) Hence net ionic equation should be written as follow:
Cd2+ + S2- ® CdS¯
1st Experiment: I nvestigate Ionic Reaction of Precipitate
PROCEDURE:
1. Place 2 ml of 1st solution into a test tube.
2. Place 2 ml of 2nd solution into another test tube.
3. Record observations of both solutions.
4. Pour the contents of one test tube into the other test tube and record observations:
· On mixing aqueous solutions of silver nitrate and sodium chloride, a white curdy precipitate of silver chloride is formed.
· On mixing an aqueous solution of barium chloride with that of copper sulphate, a white precipitate of barium sulphate is formed.
· On mixing an aqueous solution of lead nitrate with sodium sulphate, a white precipitate of lead sulphate is formed.
· On passing hydrogen sulphide gas through copper sulphate solution, a black precipitate of copper sulphide is formed.
· On adding a solution of lead nitrate to sodium iodide solution, a yellow precipitate of lead iodide is formed.
· Cobalt (II) chloride reacts with sodium carbonate to form pink/red coloured precipitate of cobalt(II) carbonate and sodium chloride.
· On adding aluminium sulphate solution to calcium chloride solution, a precipitate of calcium sulphate is formed.
5. Write the molecular, complete ionic and net ionic equations for each of the following reactions.
2nd Experiment: I nvestigate Ionic Reaction of forming a Weak Electrolyte
PROCEDURE:
The goal of this experiment is to identify seven aqueous solutions using qualitative analysis.
a. Qualitative analysis is a systematic method of recording precipitation reactions, color changes, and other visible changes to determine chemical composition.
b. Acids and bases will be identified using pH paper and phenolphthalein indicator.
| Acids | Color of universal paper indicator in acid | Base | Color of Phenolphthalein indicator in base |
| HCl | NaOH | ||
| HNO3 | NaOH | ||
| H2SO4 | NaOH |
c. After a pH analysis, all solutions will be mixed together to determine if a reaction has taken place. Write the molecular, complete ionic and net ionic equations for each of the following reactions.
d. Write the molecular, complete ionic and net ionic equations for each of the following reactions.
3rd Experiment: I nvestigate Ionic Reaction of forming an I nsoluble Gas.
PROCEDURE:
Another way a double-replacement reaction occurs if the reaction produces an unstable compound that decomposes into a gas and water.
The three most common unstable compounds which give gases are:
· Carbonic acid: H2CO3 →CO2(g) + H2O(l)
· Sulfurous acid: H2SO3→ SO2(g) + H2O(l)
· Ammonium hydroxide: NH4OH → NH3(g) + H2O(l)
· Hydrogen sulphide: 2H+ + S2- = H2S
1) Marble chips (CaCO3) react with dilute hydrochloric acid to form calcium chloride and carbonic acid. Carbonic acid is unstable and readily decomposes to form carbon dioxide and water. Since H2CO3 decomposes into CO2 and H2O, you can see bubbles forming. That's a sign that the double-replacement reaction is occurring.
· Locate the 0.1M HCl solution and the test tube with calcium bicarbonate (CaCO3). Transfer a small amount of the bicarbonate to an empty test tube. See image for amount to use.
· This time don't add any water to the sodium bicarbonate that you transferred. There is already water in the 0.1 M HCl.
· Add 0.1 M HCl to the calcium bicarbonate powder.
2) Many sulfide salts will react with acids to form gaseous hydrogen sulphide. Sodium sulfide (Na2S) react with dilute HCl acid to form sodium chloride and hydrogen sulphide, as a result you can feel the smell of rotten eggs.
By paying attention to colors and smells of gases released by a reaction one can often infer the chemical identity of the original compound. Be careful, however, many of the smells are strong and some are toxic. To smell a gas, don’t hold a solution under your nose and inhale, rather hold the solution away from you and waft the gases toward your nose with your hand.
3) Mix the following solutions in tube: ammonium chloride and sodium hydroxide. Then heat the tube. Attach to the wall test tube blue litmus paper. In the formation of white gas, look at the color of litmus paper, as a result it will be dark blue. Because ammonium hydroxide is formed, which decomposes into gaseous ammonia and water. Smell cautiously gas: ammonia has a pungent peculiar smell.
Write the molecular, complete ionic and net ionic equations for each of the following reactions.
4th Experiment: I nvestigate Ionic Reaction of dissolving the precipitate of electrolyte
PROCEDURE:
1. Place 5 ml of aluminium chloride (AlCl3) solution into a test tube.
2. Add by pipette 2 ml of 0.1N sodium hydroxide (NaOH) solution into this test tube until you will see a white precipitate of aluminium hydroxide.
3. Divide the precipitate into two test tubes.
4. Add an excess of hydrochloric acid to the first test tube, until the precipitate dissolved. Then add an excess of sodium hydroxide to the second test tube, until the precipitate dissolved too.
5. Aluminum hydroxide is amphoteric base, so it¢s precipitate react and dissolve in strong acids and alkalis. Write the molecular, complete ionic and net ionic equations for each of the following reactions.
EXERCISES:
1) Write the molecular, complete ionic and net ionic equations for each of the following reactions:
a) Aqueous solutions of sodium sulfide and calcium nitrate are mixed.
b) Aqueous solutions of barium chloride and potassium sulfate are mixed.
c) Aqueous solutions of silver nitrate and ammonium chloride are mixed.
2) Write the equation for the dissociation of the following electrolytes, call them, indicate their strength, for the weak electrolytes write the equation of the dissociation constant: a) HNO3 b) CuCl2 c) Al(OH)3
3) Write 2 example of the molecular equations for next net ionic reaction:
2 I - (aq) + Pb2+ (aq) → PbI2 (s)
4) Perform test:
| № | Question | Variant |
| 1 | The products obtained when HCl reacts with NaOH are ……………………. & ………………… respectively. Such reactions are called … | a. Cl₂, H₂O, Neutralisation b. H₂ , O₂, Combustion c. H₂O, NaCl, Double displacement d. Cl₂, H₂O, decomposition |
| 2 | What is the precipitate formed on mixing the solution of barium chloride and sodium sulphate? | a. Barium sulphate b. Barium sulphite c. Sodium chloride d. Sodium sulphite |
| 3 | Identify the product A in the reaction: Al₂O₃(s) + HClO₄(aq) --> A (aq) + H₂O (l) | a. Al(ClO₄)₃ b. Al₂(ClO₄)₃ c. Al₃ClO₄ d. AlClO₄ |
| 4 | Four students were asked to study the reaction between barium chloride and sodium sulphate. They reported their experiment as follows. Which one is a correct report ? | a. On mixing the powder of barium chloride and sodium sulphate, the colour of the mixture changes to brown. b. On adding powdered sodium sulphate to barium chloride solution; solution becomes white. c. On adding the powder of barium chloride to sodium sulphate solution; solution turns white. d. On mixing solution of barium chloride and sodium sulphate, white solid substance is formed. |
| 5 | Which of the following reactions represents the reaction between barium chloride and sodium sulphate correctly? | a. NaSO₄ + BaCl₂ → BaSO₄ + NaCl b. Na₂SO₄ + BaCl → BaSO₄ + NaCl c. Na₂SO₄ + BaCl₂ → BaSO₄ + 2NaCl d. NaSO₄ + BaCl → BaSO₄ + NaCl |
| 6 | Identify the product(s) of the following double displacement reaction CoCl₂ (aq) + Na₂CO₃ (aq) --> | a. NaCl(s) and CoCO3(s) b. NaCl(aq) and CoCO₃(aq) c. NaCl(aq) and CoCO₃(s) d. NaCl(s) and CoCO₃(aq) |
| 7 | Which of the following is not a precipitation reaction? | a. Reaction of NaNO₃ and KI b. Reaction of AgNO₃ and MgCl₂ c. Reaction of Pb(NO₃)₂ and NaI d. Reaction of FeBr₂ and Sr(OH)₂ |
| 8 | Consider the reaction Aluminium sulphate + Calcium hydroxide--> Aluminium hydroxide +Calcium sulphate. The stoichiometric ratio for the balanced equation are respectively: | a. 1:3:2:3 b. 2:1:2:1 c. 1:2:3:1 d. 1:2:1:3 |
| 9 | What is the acid formed by the reaction between lead acetate and hydrochloric acid? | a. Propanoic acid b. Formic acid c. Carbonic acid d. Acetic acid |
| 10 | Which one of the following is a neutralization reaction? | a. Reaction between ammonium chloride and KOH b. Reaction between calcium carbonate and HCl c. Reaction between NaOH and HCl d. Reaction between copper sulphate and hydrogen sulfide |
| 11 | Identify the precipitate obtained by the reaction between lead nitrate and sodium sulphate. | a. PbSO₄ b. NaNO₃ c. Pb(SO₄)₂ d. PbS |
| 12 | Which one of the following is true about a double displacement reaction? | a. Reaction between MgO and water is an example for this reaction. b. Only a single product is formed. c. Two compounds exchange ions to form new compounds. d. Electrolysis of water is an example of this reaction. |
| 13 | Which one of the following represents a double displacement reaction? | a. A + B --> C b. C --> A +B c. A + BC --> AC + B d. AB + CD --> AD + CB |
| 14 | Geethu added a small quantity of dil. HCl into a precipitate of barium sulphate in a test tube obtained by adding barium chloride solution to sodium sulphate solution. Which one of the following is the correct observation? | a. The precipitate is soluble in HCl b. The colour of the precipitate turns to yellow c. Formation of brisk effervescence d. Precipitate remains insoluble |
LABORATORY WORK
SALTS HYDROLYSIS
Objective: investigate indicators and reaction of salt ¢ s hydrolysis.
Skills to develop :
· Predict the acidity of a salt solution.
· Calculate the pH of a salt solution.
· Calculate the concentrations of various ions in a salt solution.
· Explain hydrolysis reactions.
Water molecules are highly polar and are in continuous motion. Occasionally, the collisions between water molecules are energetic enough to transfer a hydrogen ion from one water molecule to another.
Self ionization of water– the reaction in which water molecules produce ions. A water molecule that loses a hydrogen ion becomes a negatively charged hydroxide ion. A water molecule that gains a hydrogen ion becomes a positively charged hydronium ion:
HOH (l) <=> H+(aq) + OH- (aq)
In pure water at 25˚C, the equilibrium concentration of hydrogen ions and hydroxide ions are each only 1 x 10-7.
Any aqueous solution in which H+ and OH- are equal is a neutral solution:

Not all solutions are neutral. When some substances dissolve in water, they release hydrogen [H+] ions or hydroxide [OH-] ions.
If  take place in a solution this solution is called acidic
take place in a solution this solution is called acidic  .
.
If  take place in a solution this solution is called basic. Basic solutions are also known as alkaline solutions
take place in a solution this solution is called basic. Basic solutions are also known as alkaline solutions  .
.
For aqueous solutions, the product of the hydrogen ion concentration and the hydroxide ion concentration equals:  . This equation is true for all dilute aqueous solutions at 25˚C.
. This equation is true for all dilute aqueous solutions at 25˚C.
Ion-Product Constant for Water (Kw) – theproduct of the concentrations of the hydrogen ions and hydroxide ions in water: 
The quantitative designation of the acidity or alkalinity of a solution may be still further simplified by using the hydrogen ion concentration index (pH). The relation defines this index:
 and
and 
The range of the pH scale is from 0 to 14: 
 and
and 
Thus Solutions can be classified based on their pH values:
·  and pH < 7.0, the solution is acidic;
and pH < 7.0, the solution is acidic;
·  and pH = 7.0, the solution is neutral;
and pH = 7.0, the solution is neutral;
·  and pH > 7.0, the solution is basic.
and pH > 7.0, the solution is basic.


 In practice, the acidity or alkalinity of a solution is conveniently determined by means of indicators – substances that change color depending on the relative concentrations of H+ and OH- ions. This method is called a colorimetric. Thus, indicator is a halochromic chemical compound (weak organic acids or bases that react with ions in solution) that is added in small amounts to a solution so that the pH (acidity or basicity) of the solution can be determined visually. The color change of different indicators occurs at different hydrogen ion concentrations, which is important for chemical analysis.
In practice, the acidity or alkalinity of a solution is conveniently determined by means of indicators – substances that change color depending on the relative concentrations of H+ and OH- ions. This method is called a colorimetric. Thus, indicator is a halochromic chemical compound (weak organic acids or bases that react with ions in solution) that is added in small amounts to a solution so that the pH (acidity or basicity) of the solution can be determined visually. The color change of different indicators occurs at different hydrogen ion concentrations, which is important for chemical analysis.
For more precious measuring of pH the special tools are widely used– pH-meters, which provides assurance of measuring within the limits of ± 0,01.
1st Experiment. Determine the pH of water and various electrolyte solutions using acid-base indicators
Materials and Reagents :test tubes, acid-base indicators, electrolytes solutions
PROCEDURE:
1. Data Table 1 lists the formulas of the 3 electrolytes solutions you will be using.
2. Set up a test tube rack with three test tubes in a row.
3. Use the marking pen to number the test tubes to correspond to the numbers of the first indicator in Table 1.
4. Add 10 mL of each electrolyte solution to each of the test tubes.
5. Use the thin stem pipet to add 3 drops of first indicator to each test tube. Swirl to mix the drops into the solution.
6. Note the color of the indicator in each tube and enter the data into the Table 1.
| № | Indicator type | D pH range which indicator changes color | The indicator color in …. solution | ||
| Acidic (HClaq) | Neutral (H2Oaq) | Basic (NaOHaq) | |||
| 1 | Methyl orange (sln) | 3,2 – 4,4 | |||
| 2 | Blue litmus paper | 5,0 – 8,0 | |||
| 3 | Phenolphthalein (sln) | 8,2 – 10,0 | |||
| 4 | Universal Ind (sln) | 0 – 14 | |||
| 5 | Universal Ind paper | 0 – 12 | |||
Analysis:
1. Which indicator is used to determine the acidic solution?
2. Which indicator is used to determine the basic solution?
A salt is formed between the reaction of an acid and a base. Usually, a neutral salt is formed when a strong acid and a strong base is neutralized in the reaction: H+ + OH- = H2O
There are four possible ways of forming salts:
1) If the salt is formed from a strong base and strong acid, then the salt solution is neutral, indicating that the bonds in the salt solution will not break apart (indicating no hydrolysis occurred) and is neutral (pH=7).
2) If the salt is formed from a strong acid and weak base, the bonds in the salt solution will break apart and becomes acidic (pH<7) and hydrolyzes.
3) If the salt is formed from a strong base and weak acid, the salt solution is basic (pH>7) and hydrolyzes.
4) If the salt is formed from a weak base and weak acid, it will hydrolyze, but the acidity or basicity depends on the equilibrium constants of Ka and Kb. If the Ka value is greater than the Kb value, the resulting solution will be acidic and vice versa:
· If Ka(cation) > Kb(anion) the solution of the salt is acidic.
· If Ka(cation) = Kb(anion) the solution of the salt is neutral.
· If Ka(cation) < Kb(anion) the solution of the salt is basic.
Table 5 - Four possible ways of forming salts and their hydrolysis
| № | Type of salt is involved in hydrolysis | Mechanism of hydrolysis | C ations types | A nions types |
| 1 | A salt formed between a strong acid and a strong base is an neutral salt | No hydrolysis (pH=7) | Strong base c ations | Strong acid a nions |
| Na+, K+, Rb+, Cs+, Ca2+, Sr2+, Ba2+ | Cl-, Br-, I-, NO3-, SO42-, ClO4- | |||
| 2 | A salt formed between a strong acid and a weak base is an acid salt | Cationic hydrolysis (pH<7) | Weak base c ations | Strong acid a nions |
| Insoluble in water base cations: NH4+, Ag+, Cu2+, Zn2+, Al3+, Cr3+ and others | Cl-, Br-, I-, NO3-, SO42-, ClO4- | |||
| 3 | A salt formed between a weak acid and a strong base is a basic salt | Anionic hydrolysis (pH>7) | Strong base c ations | Weak acid a nions |
| Na+, K+, Rb+, Cs+, Ca2+, Sr2+, Ba2+ | F-, CH3COO-, CN-, NO2-, S2-, CO32-, SiO32-, SO32-, PO43- | |||
| 4 | A salt formed between a weak acid and a weak base can be neutral, acidic, or basic depending on the relative strengths of the acid and base. | Cationic-anionic hydrolysis (pH » 7) | Weak base c ations | Weak acid a nions |
| Insoluble in water base cations: NH4+, Ag+, Cu2+, Zn2+, Al3+, Cr3+ and others | F-, CH3COO-, CN-, NO2-, S2-, CO32-, SiO32-, SO32-, PO43- |
The reaction of salt takes place in the solution. In reality, and looking at a wider variety of 'salts', the picture is much more complicated and a 'salt' solution may be acid, neutral or alkaline depending on the nature of the interaction of the salt ions with water. The reaction of the salt with water, whereby the salt is dissociated and decomposed to form a weak electrolyte (weak acid or weak base) called hydrolysis ("chemical decomposition by water," 1880, formed in English from hydro- + Greek lysis "a loosening, a dissolution," from lyein "to loosen, dissolve"). Hydrolysis is the reverse of neutralization.
To determine acidity or basic of a salt:
1. Write the neutralization reaction in reverse (salt and water makes base and acid).
2. Break up the salt into its ions. Water is weak electrolyte and does not dissociate.
3. To write the base, add an OH- to the cation (the positive ion) for each positive (+) charge.
4. To write the acid, add H+ to the anion (the negative ion) for each negative (–) charge.
If you for a strong base, the salt is basic (NaOH, KOH, LiOH, Ca(OH)2 ...etc.)
If you form a strong acid it is acidic. (HCl, HNO3, HClO4, HBr...)
If you form both a strong acid and a strong base it is neutral.
EXAMPLES
1) SODIUM NITRITE NaNO2
NaNO2 + H2O <=> NaOH + HNO2
Strong weak
Base acid
Na+ + NO2- + H2O <=> Na+ + OH- + HNO2
NO2- + H2O <=> OH- + HNO2 (basic of a salt, pH>7)
Anion
Hydrolyzed
Since NaOH is a strong base it breaks up and yields OH-, the salt is basic. HNO2 is a weak acid and will form (does not break up in water).
2) AMMONIUM BROMIDE NH4Br
NH4Br + H2O <=> NH4OH + HBr
Weak strong
Base acid
NH4+ + Br- + H2O <=> NH3 +H2O + H+ + Br-
NH4+ + H2O <=> NH3 +H2O + H+ (acidity of a salt, pH<7)
Cation
Hydrolyzed
Since HBr is a strong acid it breaks up and yields H+, the salt is acidic. NH4OH is a weak base. They generally stay together, however this is actually breaks down into ammonia and water.
2nd Experiment. Investigate the reaction of salt hydrolysis by colorimetric method.
Materials and Reagents :test tubes, universal indicator solution and paper, salts solutions
PROCEDURE:
Identify the relative strengths (weak or strong) of the acidic ions and basic ions in solution. Predict whether the resulting solution will be acidic, basic or neutral. Use a pH meter (or pH paper) to determine the pH of each solution. Write a balanced equation for each hydrolysis reaction. Which salt is not hydrolyzed?
1. Data Table 5 lists the formulas of the four salts you will be using. Using your knowledge of hydrolysis, form a prediction about which of the salt solutions will be acidic, which will be basic, and which, if any, will be neutral. Record your prediction in the space provided.
2. Set up a test tube rack with four test tubes in a row.
3. Use the marking pen to number the test tubes to correspond to the numbers of the first four salts in the following table.
| № | Formula of salt | Structure of salt | Color of indicator in salt solution | Approximate рН value on scale indicator | The reaction medium (acidity, basic or neutral) | |
| Base (weak or strong) | Acid (weak or strong) | |||||
| 1 | ||||||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
4. Use the spatula to place a small amount of the first six salts in the corresponding test tubes 1 – 4. Put one salt in each tube. Clean the spatula with a paper towel between each use.
5. Add 10 mL of distilled water to each of the test tubes.
6. Close each test tube with a rubber stopper, and shake each tube until the solid salt is dissolved completely.
7. Remove the rubber stoppers. Use the thin stem pipet to add 3 drops of universal indicator to each test tube. Swirl to mix the drops into the solution. (Alternatively, use pH paper or pH probes to test the pH of the solutions.)
8. Use the chart of pH and indicator colors to determine the pH of each solution. Record the value in Data Table 1. Identify whether the pH is acidic, basic, or neutral in the column provided in Data Table 5.
9. All solutions except Pb(NO3)2 can be disposed of down the sink, flushing with plenty of water. Collect the lead (II) nitrate in a container provided by your teacher to be disposed of properly.
10. Repeat steps 2-9 with the last three solutions.
11. Some of the 4 salts you tested will cause water to undergo hydrolysis to form weak acids or weak bases. Other salts will not cause hydrolysis. Complete the last two columns in Data Table 5 by writing the formula for the species produced in the correct column. Refer to the background information example if you need help with this.
Analysis:
1) Write a net ionic equation to show the species formed for each salt that undergoes a hydrolysis reaction with water. Use the equation in the background information as an example. Write “NO REACTION” if the salt does not cause hydrolysis.
2) Complete the data table showing the weak acid or weak base formed in the products. Use your net ionic equations to determine this.
3) Be prepared to discuss in class:
· Which chemical salts formed acidic solutions?
· Which chemical salts formed basic solutions? How do you identify ions that come from a weak acid?
· How do you identify ions that come from a weak base?
· How do you write an equation for the hydrolysis of water?
EXERCISES:
1) What is the value of pH and pOH in solutions in which the concentration of hydrogen ions is: a) 1·10-7; b) 1·10-5; and the concentration of hydroxide ions is: c) 1·10-10 ; d) 1·10-12 mol/l?
2) What is pH value of 0,01M sodium hydroxide solution if its dissociation is complete?
3) What is pH value of 0,05N sulfur acid solution if its dissociation is complete?
4) What salt aqueous solution will have acidic medium? Write the corresponding equations:
a) Aluminum nitrate, Potassium sulfide, Potassium chloride;
b) Potassium carbonate, Iron (II) sulfate, Lithium chloride;
c) Copper (II) chloride, Sodium chloride, Lithium carbonate.
5) What salt aqueous solution will have alkaline medium? Write the corresponding equations:
a) Calcium chloride, Aluminum chloride, Sodium silicate;
b) Lithium nitrate, Potassium carbonate, Zinc nitrate;
c) Potassium chloride, Aluminum chloride, Sodium sulfide.
6) Perform test
| № | Question | Variant |
| 1 | Choose substances that form water solutions with alkaline medium: | a) Sodium chloride b) Hydrogen chloride c) Sodium carbonate d) Ammonium chloride e) Sodium nitrite |
| 2 | The medium of water solution of sodium hydrogen phosphate is: | a) Slightly acidic b) Strongly acidic c) Slightly alkaline d) Strongly alkaline e) Neutral |
| 3 | Note the solution that has most alkaline condition: | a) [H+] = 10-7 mol/l b) [OH-] = 5×10-8 mol/l c) [OH-] = 10-4 mol/l d) [OH-] = 5×10-10 mol/l e) pH = 5,0 |
| 4 | The pH of a solution is equal to 5,0. Find the hydrogen ion concentration: | a) 1,0×10-14 b) 1,0×10-9 c) 1,0×10-5 d) 5,0 e) 9,0 |
| 5 | Calculate the pH of a acetic acid solution, if its dissociation degree is equal to 1%: | a) 0,76 b) 2,76 c) 3,76 d) 4,76 e) 5,76 |
| 6 | What medium will be in a solution of aluminium nitrate: | a) Alkaline b) Neutral c) Acidic d) none of these |
| 7 | Find compounds that reduce a degree of hydrolysis of a sodium cyanide solution: | a) Nitrate of potassium b) Water c) Sodium hydroxide d) Barium chloride e) Potassium hydroxide |
| 8 | Hydrolysis of the salt causes acidic reaction of medium in water solution: | a) BaF2 b) ZnCl2 c) Na2S d) CH3COONH4 e) Na2CO3 |
| 9 | The hydrogen ion concentration in electrolyte solution with pOH=9 is equal to: | a) 1,0×10-14 mol/dm3 b) 1,0×10-5 mol/dm3 c) 5,0 mol/dm3 d) 9,0 mol/dm3 e) 1,0×10-9 mol/dm3 |
| 10 | Where is the hydrolysis not possible: | a) NaCl b) FeCl3 c) Al2(SO4)3 d) K2SO4 e) NH4NO3 |
| 11 | Note the ions that can not be in considerable amounts in a neutral solution: | a) Ba2+ b) CO32- c) NO3- d) Li+ e) SO42- |
| 12 | Where does the acid base indicator phenolphthalein gain of crimson colour: | a) HCl b) KOH c) Na2CO3 d) NaCl e) H2CO3 |
| 13 | The alkaline solution can be received at dissolution in water of: | a) Aluminium sulphate b) Sodium hydrogen phosphate c) Sodium dihydrogen phosphate d) Iron (III) chloride e) Ammonium chloride |
| 14 | The water solution of the following compounds have acidic medium: | a) Sodium chloride b) Copper sulphate c) Sodium carbonate d) Ammonium chloride e) Sodium sulphide |
| 15 | Find basis and acid that form salt XA, if such ionic - molecular equation A- + H2O = HA + OH- correspond to its hydrolysis: | a) Strong acid and weak base b) Weak acid and strong base c) Weak acid and weak base d) Strong acid and strong base |
LABORATORY WORK
WATER HARDNESS
Objective: to determine in laboratory a) by titration with 0,1N HCl solution temporary water hardness and b) by titration with 0,05N EDTY solution general water hardness
Skills to develop:
· to be able to titrate
· to prepare solutions
· to measure the hardness of the water
Hard water is water that has high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of calcium and magnesium-containing minerals such as limestone, chalk and dolomite.
Hard drinking water is generally not harmful to one's health, but can pose serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling towers, and other equipment that handles water. In domestic settings, hard water is often indicated by a lack of suds formation when soap is agitated in water, and by the formation of lime scale in kettles and water heaters:
CaSO4 + 2RCOONa = (RCOO)2Ca¯ + Na2SO4
MgCl2 + 2RCOONa = (RCOO)2Mg¯ + 2NaCl
Water's hardness is determined by the concentration (mmol-eq/L) of multivalent cations (Ca2+ and Mg2+) in 1 liter of the water. The total water hardness is the sum of the molar concentrations of Ca2+ and Mg2+, in mmol/L units. One unit of hardness (1 mmol/L) corresponds to the content of calcium ions equal to 20,04 mg / L of magnesium ions or equal to 12,16 mg / liter:

Theoretically any water hardness (H) can be calculated by the formula:

On scale hardness distinguishes soft, hard and very hard natural water:
| Value of hardness (mmol/L) | Water hardness | Example |
| H > 1,5 | Very soft | Most soft water is rain, snow (0.1 mmol/L) |
| H = 1,5 – 4,0 | Soft | Rain water |
| H = 4,0 – 8,0 | Moderately hard | Drinking water |
| H = 8,0 – 12 | Hard | |
| H > 12,0 | Very hard | The most hard - water of oceans (up to 130 mmol/L) |
General hardness is the sum of temporary and permanent hardness: GH = TH + RH
Temporary (carbonate) hardness is a type of water hardness caused by the presence of dissolved bicarbonate minerals: Ca(HCO3)2 and Mg(HCO3)2. Temporary hardness can be reduced either by boiling the water, or by the addition of lime (calcium hydroxide) through the softening process of lime softening. Boiling promotes the formation of carbonate from the bicarbonate and precipitates calcium carbonate out of solution, leaving water that is softer upon cooling:

Unit of measure carbonate hardness: in German degrees of hardness. 1AdH corresponds to 18 mg/L CaCO3:
· below 10 AdH – soft
· from 20A dH – hard
· above 30A dH – very hard
Permanent hardness is hardness (mineral content) that cannot be removed by boiling. When this is the case, it is usually caused by the presence of calcium sulfate and chloride (CaSO4, CaCl2), magnesium sulfates and chloride (MgSO4, MgCl2) in the water, which do not precipitate out as the temperature increases. Ions causing permanent hardness of water can be removed by using a water softener, or ion exchange column. It can be removed by soda (Na2CO3), surfactants (Na3PO4) or lime (Ca(OH)2):

Water hardness can be determined by the following 2 methods:
· By titration with 0,1N HCl solution in the presence of a titration acid-base indicator methyl orange. In this method temporary hardness can be measured.
· By titration with disodium edetate 0,05N Na2H2Y solution (Trilon B) in the presence of a titration indicator Eriochrome Black-T. It is a salt of EDTA (ethylenediaminetetraacetic acid). In this method general hardness can be measured.

Molecular weight of disodium salt of EDTA:
(CH2COOH)2N2(CH2)2(CH2COONa)2*2H2O = (12+1*2+12+16*2+1)×2 +14*2+ (12+2)*2+ (12+1*2+12+16*2+23)×2 + 2*18 = 118+ 28+28+162+36 = 372 g/mol
EDTA has a greater affinity for Ca2+ and Mg2+ when it is in the form of the dihydrogen anion H2EDTA2-. This is the ionic form of EDTA at pH 10. H2EDTA2- binds to a Ca2+ ion by forming four special covalent bonds are called coordinate covalent bonds:

In today’s experiment, you will determine the total concentration of calcium and magnesium ions in a hard water sample using EDTA in solution ammonia buffered (NH4OH – NH4Cl) to a pH of 10.
Preparation of ammonia buffer solution: 145 ml of liquor ammonia (NH4OH) of specific gravity 0.88 + 15 gm NH4Cl + distilled water to make 250 ml solution to give a pH of 10.
Erio - T indicator or Eriochrome Black-T indicator is used in this titration. When it is chealted or acidifies, it produces a pink red solution. When it is not chelated and under basic conditions it is blue.
 A titrationis a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required to bring about a given effect in reaction with a known volume of the test solution in the titration flask. A buret is used to deliver the second reactant to the flask and an indicator or pH-meter is used to detect the endpoint of the reaction.
A titrationis a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required to bring about a given effect in reaction with a known volume of the test solution in the titration flask. A buret is used to deliver the second reactant to the flask and an indicator or pH-meter is used to detect the endpoint of the reaction.
This method relies on observing a color change in the solution. Indicators are weak organic acids or bases that are different colors in their dissociated and undissociated states. Because they are used in low concentrations, indicators do not appreciably alter the equivalence point of a titration. The point at which the indicator changes color is called the end point. The volume added to achieve the end point may be calculated using this formula (equivalent law):

 Begin by preparing your buret, as described on the buret page. Your buret should be conditioned and filled with titrant solution. You should check for air bubbles and leaks, before proceding with the titration.
Begin by preparing your buret, as described on the buret page. Your buret should be conditioned and filled with titrant solution. You should check for air bubbles and leaks, before proceding with the titration.

 Prepare the solution to be analyzed by placing it in a clean Erlenmeyer flask or beaker. If your sample is a solid, make sure it is completely dissoloved. Put a magnetic stirrer in the flask and add indicator.
Prepare the solution to be analyzed by placing it in a clean Erlenmeyer flask or beaker. If your sample is a solid, make sure it is completely dissoloved. Put a magnetic stirrer in the flask and add indicator.

 Use the buret to deliver a stream of titrant to within a couple of mL of your expected endpoint. You will see the indicator change color when the titrant hits the solution in the flask, but the color change disappears upon stirring.
Use the buret to deliver a stream of titrant to within a couple of mL of your expected endpoint. You will see the indicator change color when the titrant hits the solution in the flask, but the color change disappears upon stirring.
Approach the endpoint more slowly and watch the color of your flask carefully. Use a wash bottle to rinse the sides of the flask and the tip of the buret, to be sure all titrant is mixed in the flask.

 As you approach the endpoint, you may need to add a partial drop of titrant. You can do this with a rapid spin of a teflon stopcock or by partially opening the stopcock and rinsing the partial drop into the flask with a wash bottle. Ask your TA to demonstrate these techniques for you, in the lab.
As you approach the endpoint, you may need to add a partial drop of titrant. You can do this with a rapid spin of a teflon stopcock or by partially opening the stopcock and rinsing the partial drop into the flask with a wash bottle. Ask your TA to demonstrate these techniques for you, in the lab.
 Make sure you know what the endpoint should look like. For phenolphthalein, the endpoint is the first permanent pale pink (purpule). The pale pink fades in 10 to 20 minutes.
Make sure you know what the endpoint should look like. For phenolphthalein, the endpoint is the first permanent pale pink (purpule). The pale pink fades in 10 to 20 minutes.
If you think you might have reached the endpoint, you can record the volume reading and add another partial drop. Sometimes it is easier to tell when you have gone past the endpoint.

If the flask looks like this, you have gone too far!

When you have reached the endpoint, read the final volume in the buret and record it in your notebook.
Subtract the initial volume to determine the amount of titrant delivered. Use this, the concentration of the titrant, and the stoichiometry of the titration reaction to calculate the number of moles of reactant in your analyte solution.
1st Experiment. Determine the Temporary Hardness of Water by Acid-base titration
Materials and Reagents : 0.1N hydrochloric acid (HCl) solution, acid-base indicator methyl orange, Erlenmeyer flasks (250 ml), Graduated cylinder (100 ml), buret (25 ml).
PROCEDURE:
For determining temporary hardness, we measured by cylinder 100 ml hard water and taken into a 250 ml conical flask. Then few 3-4 drops of methyl orange is added in it as an indicator. Now titration is carried out by adding 0.1N cold HCl until the yellow color of methyl orange turns red. Titration was repeated 3 times
During titration in solution occurs the following reaction:
Ca(HCO3)2 + 2HCl = CaCl2 + H2O + CO2
Mg(HCO3)2 + 2HCl = MgCl2 + H2O + CO2
Protocol of analysis:
1. The volume of water taken for titration 
2. Volume of acid which went on titration for the first time 
3. Volume of acid which went on titration for the second time 
4. Volume of acid which went on titration for the third time 
5. The average amount of acid which went on titration 
6. Normality of acid solution NHCl = 0,1N
 Calculate TH (mmol/L) by following formula:
Calculate TH (mmol/L) by following formula:
2nd Experiment. To determine the General Hardness of Water by Complexometric titration
Materials and Reagents : 0.05N disodium salt of EDTA solution, complexometric indicator Eriochrome Black-T, ammonia buffer solution, Erlenmeyer flasks (250 ml), Graduated cylinder (100 ml), buret (25 ml).
Procedure:
· Use a graduated cylinder to dispense 50.00 mL of analyses water into a 250 mL flask.
· Add 2 mL of pH 10 ammonia buffer solution, and then 7 - 10 drops of Eriochrome Black T indicator. The resulting solution will turn the dark red color.
· Titrate the solution with 0,05N EDTY from your buret. As you near the endpoint, the solution will turn purple. Continue to slowly add EDTY until the solution turns blue, with no trace of red.
Protocol of analysis:
1. The volume of water taken for titration 
2. Volume of EDTA which went on titration for the first time 
3. Volume of EDTA which went on titration for the second time 
4. Volume of EDTA which went on titration for the third time 
5. The average amount of EDTA which went on titration 
6. Normality of acid solution NEDTY = 0,05N

Calculate GH (mmol/L) by following formula:
EXERCISES:
1) The total hardness of water is equal to 5.25 mmol / L. Calculate how many grams of sodium phosphate should be added to 500 liters of water to remove its hardness.
2) Calculate the water hardness when in 1 liter of water was dissolved 40.04 mg of calcium ions and 24.32 mg magnesium ions.
3) Determine what is the temporary hardness of the water, if the titration of 20 ml of water was spent on average 5.25 ml of 0.1 N hydrochloric acid solution?
Дата добавления: 2018-09-20; просмотров: 643; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
