Solutions of Solids in Liquids
Solutions of this type are most common. In solutions of solids in liquids, the liquid is invariably referred to as a solvent and the solid dissolved in it as the solute. If a solute is added in small amounts at a time to a given amount of a solvent at a constant temperature, with vigorous stirring of the solvent after each addition, a stage is reached when the added solute no more disappears, i.e. goes into solution but remains undissolved. The solution is then said to be saturated. A solution which remains in contact with undissolved solute is termed as saturated. It can also be defined as one which is in equilibrium with the excess of solid at a particular temperature.
The amount of solute dissolved in 100g of a solvent to form a saturated solution at a given temperature is termed the solubility of the solute in the given solvent at that temperature. Each substance has a characteristic solubility in a given solvent at a definite temperature.
When a solid is added to the solvent, the particles from the solid diffuse into it. The solute and solvent molecules move constantly in the solution phase. Some of the particles of the solute return to the solid state due to collisions. Thus, two opposite processes operate simultaneously.
Dissolution: Particles of solute leaving the solid and dissolving in the solvent.
Recrystallisation: Solute particles returning to the solid form.
When these two processes move with the same speed, an equilibrium stage is reached.
Solute (solid) 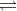 Solute (dissolved)
Solute (dissolved)
Thus, a dynamic equilibrium exists in a saturated solution. When a saturated solution prepared at a higher temperature is cooled, it gives a solution which contains usually more of solute than required for the saturated solution at that temperature. Such a solution is referred to as a supersaturated solution. It is usually unstable and changes to saturated solution when excess of solute comes out in solid state.
The following factors influence the solubility of a solid in a liquid :
· Nature of solute : The solutes (solids) can be classified as ionic and non-ionic solids. The ionic solids consist of positively and negatively charged ions. It is the force of attraction between the ions, i.e., lattic energy which opposes the tendency of a solute to dissolve. This force of attraction is different in different ionic solids depending on the charges present on the ions and distance between ions (ionic radii). The ionic solutes having high less lattice energy have more solubility. The ions are solvated by the solvent molecules and in this process energy (known as hydration energy) is released. When the hydration energy is high, the ionic solid is more soluble.
|
|
|
Many non-ionic substances dissolve in polar solvents due to hydrogen bonding. Generally, if the solute and solvent have similar characteristics, i.e. both polar or both non-polar, the solubility is high and if both are dissimilar, the solubility is found low.
· Nature of solvent: Ionic solids dissolve to a larger extent in a solvent having a high dielectric constant as compared to solvents of low dielectric constants. Dielectric constant of water is 80 while that of methyl alcohol is 33.5 An ionic solid, therefore, dissolves more readily in water than in methyl alcohol. Benzene has a very low dielectric constant of 2.3 and, hence, ionic solids do not dissolve in benzene.
For non-ionic solids, the guiding principle is ‘like dissolves like, i.e., if the solvent is polar, it will dissolve the polar solutes and if it is non-molar, it will dissolve the non-polar solutes in it.
· Temperature: The solubility of a solute in a given solvent varies appreciably with temperature.
It is observed that the solubility of NaCl increases very slightly with an increase in temperature whereas those of KNO3, NaNO3, AgNO3, KI, etc., increase greatly. A sharp break in a solubility curve indicates the formation of a compound whose solubility is different from that of the substance from which it has been formed as in the case of Na2SO410H2O. It losses its water of crystallization at 32.3°C and is converted into anhydrous form. There are few substances like calcium acetate, cerium sulphate, calcium chromate etc., which show a decrease in solubility with rise in temperature.
Generally, solubility depends on heat of solution. If a substance dissolves with absorption of heat, the solubility increases with rise of temperature. On the other hand, if a substance dissolves with evolution of heat, the solubility decreases with rise of temperature.
Дата добавления: 2018-09-20; просмотров: 273; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
